It was late January of my first Wisconsin winter. I had started my Ph.D. studies at the University of Wisconsin-Madison about half a year ago. I had just failed the qualifying exam — a tortuous two day event with a cumulative 6 hours of written exams covering a wide range of topics to determine qualification to receive a Ph.D. in Mechanical Engineering. I had been told that I should seriously consider a job in industry. My dreams of combining what came natural to me in teaching with the fun of research had shattered.
I don’t know why, but when I was walking by the mail room one day I decided I should check my mailbox. I had never received anything. But today was different, a letter not to any of the other graduate students with an L for the last name, but one for me. I opened it.
The hair stood up on the back of my neck, my knees started shaking, and tears welled up in my eyes.
On the letter was typed a congratulations that I had won the Western Association of Graduate Schools Distinguished Thesis Award for 2007.
The Western Association of Graduate Schools consisted of 85 member institutions in the Western United States and Canada. The award covered all disciplines. Holy cow! Spurred by this momentum I passed my next qualifying exam, raced through my Ph.D. in experimental cryogenics in just 3 years, and skipped a post-doctoral position entirely to land my current spot on the faculty at Washington State University — just 40 minutes from where I grew up… Life has it’s ups and downs.
Thermodynamic Surfaces for Fluids
It’s still astonishing to me that anyone could understand my thesis, let alone consider it for an award. The title, “Fundamental Equations of State for Parahydrogen, Normal Hydrogen, and Orthohydrogen,” gives an idea for how technical it was. With help from my advisors and researchers from the National Institute for Standards and Technology (NIST), I developed new equations that expressed all of the thermodynamic properties of liquid and vapor hydrogen. The equations were soon adapted as standards for hydrogen vehicle refueling. Considering the global exchange of hydrogen is a multi-billion dollar industry, and the flammability dangers, having very accurate equations was important.
Another way to think about my thesis is to consider equations of state as topographical maps — the kind that show elevations to help you to navigate a mountain on a backpacking trip. Equations of state connect all of the thermodynamic properties in a single equation that fits the ‘mountain’ that is the fluid surface — and all fluids have one. Here are a couple of plots from my thesis. The plot on the left shows the “mountain” that divides the vapor from the liquid region, with phase change in between. Above the mountain are the experimental measurements of pressure-density-temperature, in this case taken at constant density. The plot on the right shows lines of constant temperature predicted by the equation of state I developed. This plot also includes the melting line that separates solid from fluid. From graphs like these you could engineer a system to run hydrogen at any practical temperature, pressure, or density.
Each surface is unique to each fluid. The phase change ‘mountain’ is very different for hydrogen than water, or mercury. Despite over 150 years of effort, we still do not have purely theoretical equations that can accurately model these surfaces to the precision of experimental measurements. My equations, like most of the leading equations, are simply numerical fits of the surface. This is an important point. Most scientists and engineers are doggedly determined to derive equations from basic fundamentals, yet the most encompassing laws of physics still rely on these empirical fits for fluid data. Another way to think about it, atoms and molecules are incredibly complex. Trying to mathematically model all of the ways they can interact in order to accurately predict the bulk properties would require such a complex set of equations they wouldn’t be worth solving. Social space is likely no different.
In the late 1800’s, thermodynamic researchers were trying to develop these “surfaces of state” for any simple fluid, even water. Around this same time, Kelvin, Maxwell, Helmholtz, and Gibbs were all trying to merge and resolve the first law of thermodynamics (balancing energy), and the second law of thermodynamics (balancing entropy). It was Gibbs, working in near isolation at Yale, that had the spatial-graphical understanding to merge the two laws with the fluid surface.
Gibbs and the Energy for Change
I’ve taught graphical plotting of thermodynamic properties for over a decade now. It wasn’t until I went back and read Gibbs’ original paper from 1873 “Representation By Surfaces of the Thermodynamic Properties of Substances” that I realized the significance of these methods to the history of thermodynamics. By 1873 it was well established that the useful work production from a substance was related by the change in U + Pv, what was also known was the degradation of this useful work by the change in TS. As Gibbs began trying to relate these to the property surface for water, he realized that the change in the combination of the properties U + Pv – TS was 0 while the fluid was stationary, known as equilibrium, and negative when the fluid was spontaneously changing phase at constant temperature and pressure. He and Maxwell developed the following figures based on this realization:
James Clerk Maxwell famously molded the surface from plaster and mailed copies to his scholarly friends to aid in visualizing the phase change problem. The figure in the middle is particularly useful. In this original figure, drawn at a single value of volume (density), η is entropy (S), ε is internal energy (U), and A is the original state of the fluid (at any given point it has a single value for internal energy and entropy). From this, Gibbs realized that the line MN represents the total amount of energy that would be dissipated as heat if you wasted the potential to do something, AB is the maximum amount of work available (also known as exergy, or Helmholtz energy in this context) if no entropy is generated, AC is the maximum amount of entropy that could be generated without changing internal energy or density.
Extending Gibbs energy beyond thermodynamics
Gibbs had a pretty good idea for the significance of his new property. Ultimately he could predict phase change throughout nature, not only fluids but solid materials, biological materials, and more. In 1891, Irving Fisher, one of Gibbs’ Ph.D. students finished a dissertation titled “Mathematical Investigations in the Theory of Value and Prices” that drew a direct analogy between Gibbs’ equilibrium in physical and chemical systems and the equilibrium of financial markets in social systems. The work ultimately influenced Nobel Laureate Paul Samuelson who published a seminal treatise, “Foundations of Economic Analysis” which heavily utilized principles of equilibrium, vector analysis, and probability. The Physicist and economist Robert Ayres has completed extensive work on thermodynamic equilibrium theories in economic systems. If we invented economics and money to aid in social exchanges, and invented economic properties like inflation to cope with physical phenomena like entropy, it’s not a far extension to imagine how these same thermodynamic laws directly transfer to social space.
Gibbs Energy in Social Space
Once I had equated empathy with social entropy, the question became how to represent the other common thermodynamic properties in social space. In April 2016 I had an epiphany one morning and just listed all of the properties off.
Internal Energy (U) –> value evolution
The values a society aspires towards (whether adopted or imposed) are directly related to the spiral memes that correspond to how the society transfers knowledge. The more evolved the value set, the higher the degree of plasticity and movement between value sets that is possible. For example, the Gross Domestic Product (GDP) is how the US and most Western societies gauge progress and fits in with the Performance v-Meme. The Gross National Happiness Index (GNHI) is the metric that Bhutan adopted to gauge progress and the sustainable values necessary for this small nation fall primarily into the Communitarian v-Meme, but yet require a modest GDP to achieve. The desires/perceived needs by society are also relative to a reference state, the GDP of the US is very different from Japan, etc. Just like entropy, internal energy meters don’t exist and change is relative. But change does indicate momentum, whether for better or worse.
Temperature (T) –> Energy/resources
Temperature is defined as the statistical average speed of particles in a system. As the temperature goes up, the more energy modes that can be awakened.
As temperature approaches absolute zero, molecules (people) cannot do anything but worry about survival and quantized (think mirroring) behavior dominates. This is the lowest Sprial vMemes structure above. Think a homeless person here, while at first glance you think they have the most freedom, the reality is they don’t have the resources to do anything.
With sufficient temperature enough energy/resources are available to energize more complex storage and communication modes.
Heat Capacity (Cv) -> Capacity for conceptual understanding of a topic
Cv is defined as being equal to the change in internal energy du divided by the change in temperature dT at constant volume. If a person has little to no values related to the concept in question, it takes considerable resources to significantly build and influence their opinions about it. You have to first form a core value, then build up the complexity of the value to influence it.
Density (1/v) –> Population/agent density
The tighter the packing, the faster the phase change. It is true that ideal gases don’t interact with the other particles. Rural communities and isolationists suffer similar problems.
Pressure (P) –> Stress
If a stress is being applied to a community, it increases the flow enthalpy of the system to change/work, or even eventually explode.
Enthalpy (H) –> Agency
Enthalpy is defined as H= U+Pv, which when transferred to social space becomes the value set of a person (U) plus the stress and space (v) within which a person can work. Un-empathetic people have had the agency to act throughout history because they had the values, and sufficient stress combined with the space (freedom) to act.
Helmholtz energy (A) –> Maximum work that can be produced at constant entropy
The Helmholtz energy is defined as A=U-TS. In other words, have a strong value set with no resources or empathy. You become a conduit through which things efficiently flow with minimal entropy generation. This is the prime target of authoritarians and other low v-Meme individuals. The more information you collect about the situation (Q) and empathy you develop for the problem (S) the more difficult it becomes to enact the exchange. In traditional thermodynamics, the Helmholtz energy is the “useful work” that can be transferred from a system with constant temperature and volume. In other words, this is the goal of the “anti-empathy” movement — rational individuals that work hard to avoid empathizing with others due to the siphoning of resources involved.
Gibbs Energy (G) –> Potential for societal phase change
The Gibbs Energy is defined as:
G = U + Pv – TS.
Gibbs Energy describes the potential for a system to change. If the change in Gibbs free energy is negative, a system will spontaneously change. If positive, the system requires additional inputs to drive the change. Another way to look at this, the Gibbs energy of a saturated liquid and vapor (both on the verge of phase change) is identical. So if the Gibbs energy is the same for two different social states, change is already happening! The more negative the delta becomes, the more likely a cavoom, or disruptive phase change. But think about it — the perceived progress, stress, and density of a population is counterbalanced by the available energy/resources and the empathy of the group. As Chuck says, “There has never been an evolved democracy that has declared war on another evolved democracy.”
Keep in mind what we can actually measure about a society: population density, energy/resources, and may’be stress (happiness?). This is similar to what we can measure (T,rho,P) of thermodynamic systems. U, H, A, G, and S are relative to reference states.
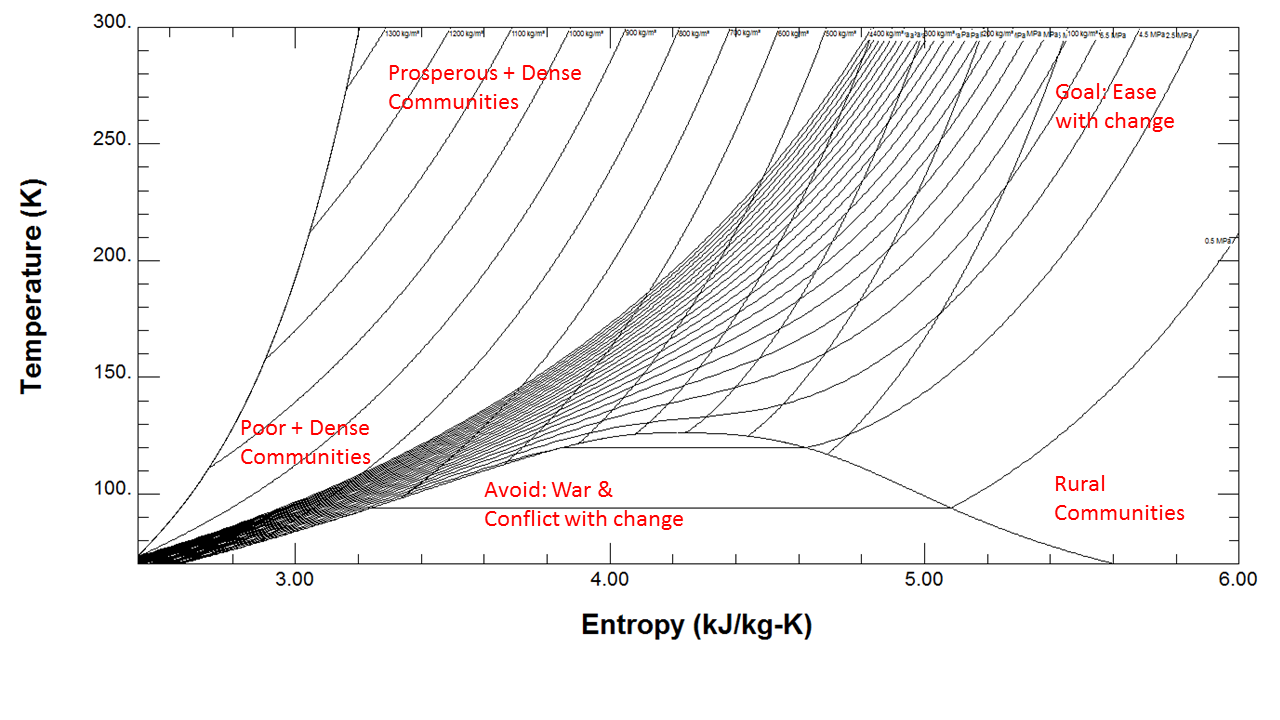
The higher the energy (temperature) and empathy (entropy) of a system the smoother the changes and the less likely you are to have a disruptive flash phase change from liquid to vapor or vice-versa. See the Temperature vs. Entropy plot below generated from Nitrogen. Density increases as you go up and to the left along with pressure (stress), the opposite direction of entropy (empathy). The area to avoid is the lower left. The area we want is the upper right. Drawing these lines based on the physics/thermodynamics of real human systems is the challenge.
Others Connecting Gibbs Energy to Social Space
Attempting to merge the concept of Gibbs equilibrium in social sciences has been a common theme of physical-chemists. Frederick Rossini received the Priestley Medal in 1971 and offered an address, “Chemical Thermodynamics in the Real World“. Rossini equated the change in entropy to individual freedoms and the change in enthalpy over temperature to security.
Sociology Professor Kenneth D. Bailey in his book “Social Entropy Theory” used traditional thermodynamic arguments, and a traditional view of entropy to conclude that society was doomed to chaos. Physical Chemist, and former president of Illinois State University, Thomas P. Wallace’s book “Wealth, Energy, and Human Values: Dynamics of Decaying Civilizations from Ancient Greece to America,” took a similar dismal view.
Chemical Engineer Libb Thimms has compiled an incredibly sophisticated wiki, titled the Encyclopedia of Human Thermodynamics, of the historical works attempting to merge social systems and humans to thermodynamics. Libb concludes that humans actually are molecules, a complex 23 atom molecule. As LIbb’s cohort has shown, many have attempted, in many ways, to apply thermodynamics to social systems.
I found most of these other attempts to model the thermodynamics of social systems after I had the initial combination of internal energy as values and empathy as social entropy. Both of those connections appear to be new to this space. So I leave the following applications, and the determination of the veracity of this approach to you.
(Note: this post is one chapter of what could become a book someday. The other chapters can be found here: https://hydrogen.wsu.edu/dr-jacob-leachman/ )